At the University of Pittsburgh, I work as a technical writing consultant. When I started, my main focus was documenting IT process integrations for the Department of Philanthropic and Alumni Engagement. However, as my role evolved, it became clear that there was a larger need—not just to write integration documentation but also to develop a document management system to organize and control an expanding collection of technical documents. Recognizing this gap, I recommended implementing a Quality Management System framework to streamline documentation processes. To address this, I proposed several key initiatives as essential operational milestones:
- Develop and Create Initial Set of PAE Technical Documents (Work Instructions) and associated QSD documents (Quality Manual, Records, Forms, Templates).
- Establish SharePoint Documentation Management System (DMS) - Build a scalable QSD Documentation Management System using our internal SharePoint assets as a Document Management Solution (DMS).
- Implement a QMS Framework for Management of PAE Technical Documentation - Support the implementation of a scalable documentation framework based on a "specialized adoption" of QMS (Quality Management System) standards, guidelines, and principles.
Duties and Responsibilities
In this role, I perform the following duties:
- Manage Document Control: Oversee the control of all Quality Management System (QMS) documents, including policies, procedures, work instructions, forms, and records.
- Create and Revise Documents: Collaborate with departments to create, update, and review QMS documents to reflect process changes, standards, and regulations.
- Ensure Compliance: Ensure QMS documentation complies with quality standards, industry regulations, and legal requirements, staying updated on changes.
- Distribute Documents: Manage document distribution to ensure accessibility, version control, and removal of obsolete documents.
- Coordinate Training and Awareness: Coordinate training programs and awareness campaigns to educate employees on the importance and use of QMS documentation.
- Maintain Records: Manage records related to quality management, such as customer complaints, corrective actions, audits, and inspections.
- Facilitate Document Retrieval: Establish efficient systems for retrieving QMS documents, especially during audits, inspections, or inquiries.
- Support Audits and Inspections: Assist in QMS audits and inspections by providing access to relevant documentation and addressing findings.
- Drive Continuous Improvement: Identify and implement process improvements within the QMS documentation management process.
- Implement Change Control: Implement a change control process to manage changes to QMS documents effectively.
- Mitigate Risks: Assess and mitigate risks associated with QMS documentation processes.
- Foster Communication and Collaboration: Collaborate with various stakeholders to align QMS documentation with business goals.
- Track Documentation Metrics: Track and report KPIs related to QMS documentation management.
- Provide Documentation Training: Provide guidance and training on effective QMS documentation management.
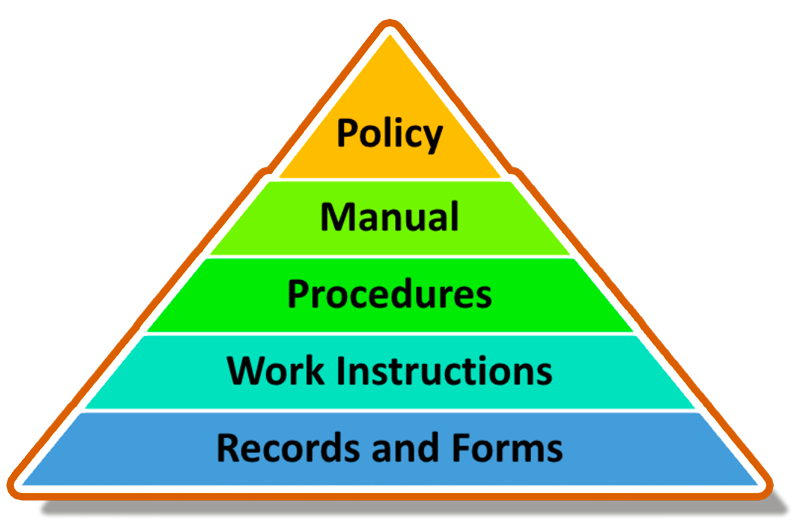
QMS Documentation Dimensions and Hierarchy
The QMS documentation can be represented as a hierarchy, as shown in this diagram.
What is QMS Documentation?
QMS documentation consists of various types of documents that outline the policies, procedures, and records necessary to implement and maintain a quality management system. This documentation can include, but is not limited to:
- Quality Policy: A statement of the organization’s commitment to quality and its goals for achieving customer satisfaction.
- Quality Manual: A high-level document that describes the structure of the QMS, including its scope, processes, and interaction of key elements.
- Procedures: Detailed instructions on how specific activities and processes are to be carried out.
- Work Instructions: Step-by-step guides for performing specific tasks or operations.
- Quality Plans: Documents outlining how quality will be managed for specific projects or products.
- Records: Evidence that processes have been followed and objectives have been met.
Ready to Elevate Your Quality Standards For Doucmentation?
Don't let complexity hold you back. Contact Scratch Writing today and take the first step towards a streamlined, lightweight, and flexible Quality Management System (QMS) for your oganizational document management. Our experts are ready to guide you through every phase of development.
Get Started With Your QMS Documentation Today
